
A safe, effective solution for adolescent depression.
NeuroStar® TMS is a non-drug, FDA-cleared adjunct treatment for ages 15-21.
Learn More
NeuroStar® TMS Works When Antidepressants Don’t
For patients who haven’t seen adequate relief from antidepressants, trying another medication isn’t always the answer. NeuroStar may be the best option: a non-drug depression treatment proven to transform lives. Find out how you can become a NeuroStar provider.
Contact Us
Are You Offering Your Depression Patients Every Option?
67% of patients with MDD aren’t happy with their current treatment or quality of life and are actively searching for new solutions.10,14,26 If antidepressants haven’t worked for your patients, don’t they deserve another option?
Offer NeuroStar Today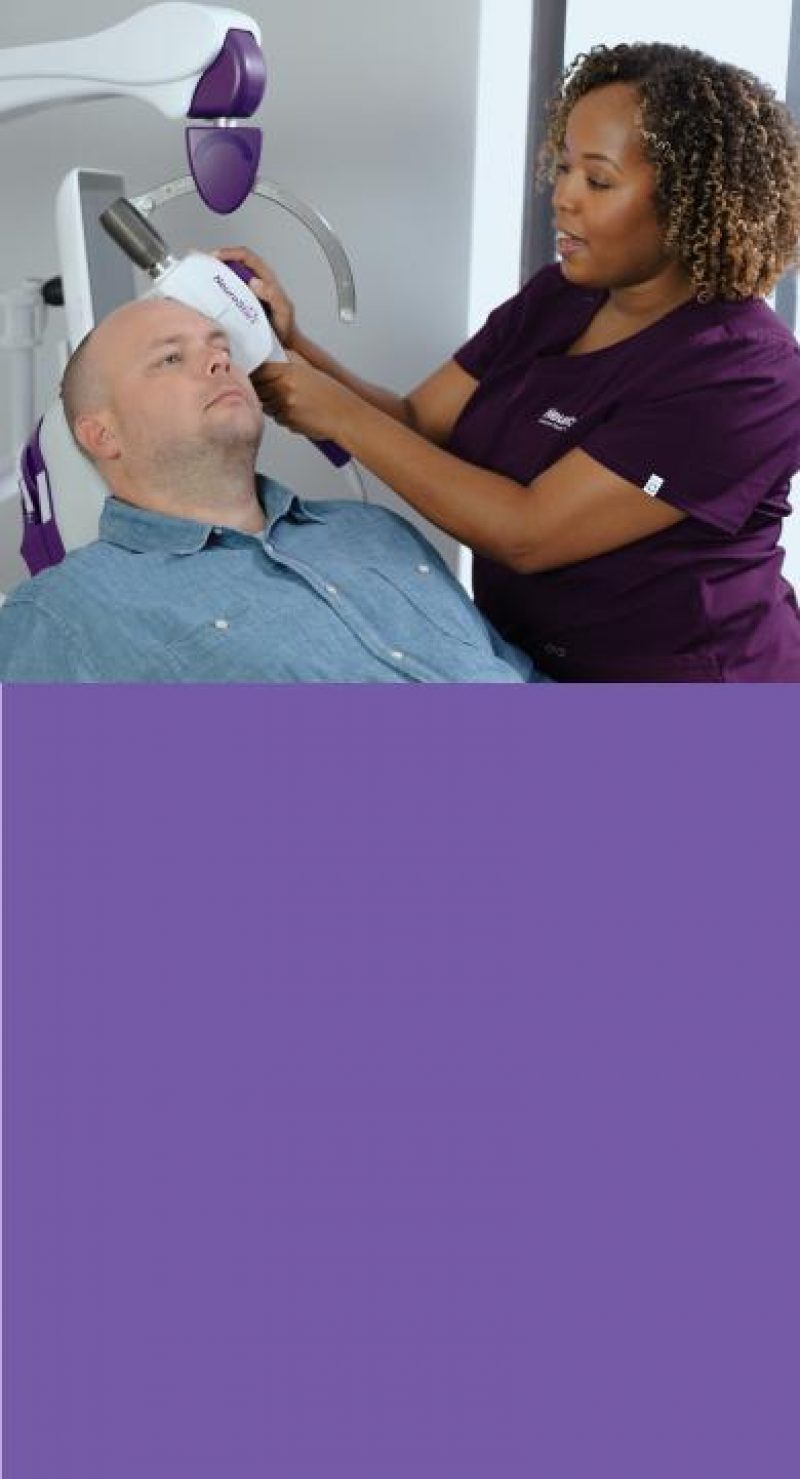
Tap Into a New Possibility for Your Patients and Your Practice
We know you care about your patients, and you’re also running a business. NeuroStar sets you up for success on both fronts, with the alternative your drug-resistant depression patients need, and the resources to help your practice thrive
View Practice Benefits
Experience Matters
NeuroStar is the market leader in TMS clinical practice, with over 1,100 practices offering NeuroStar, and more than 100 NeuroStar supporting team members.
See What Sets Us Apart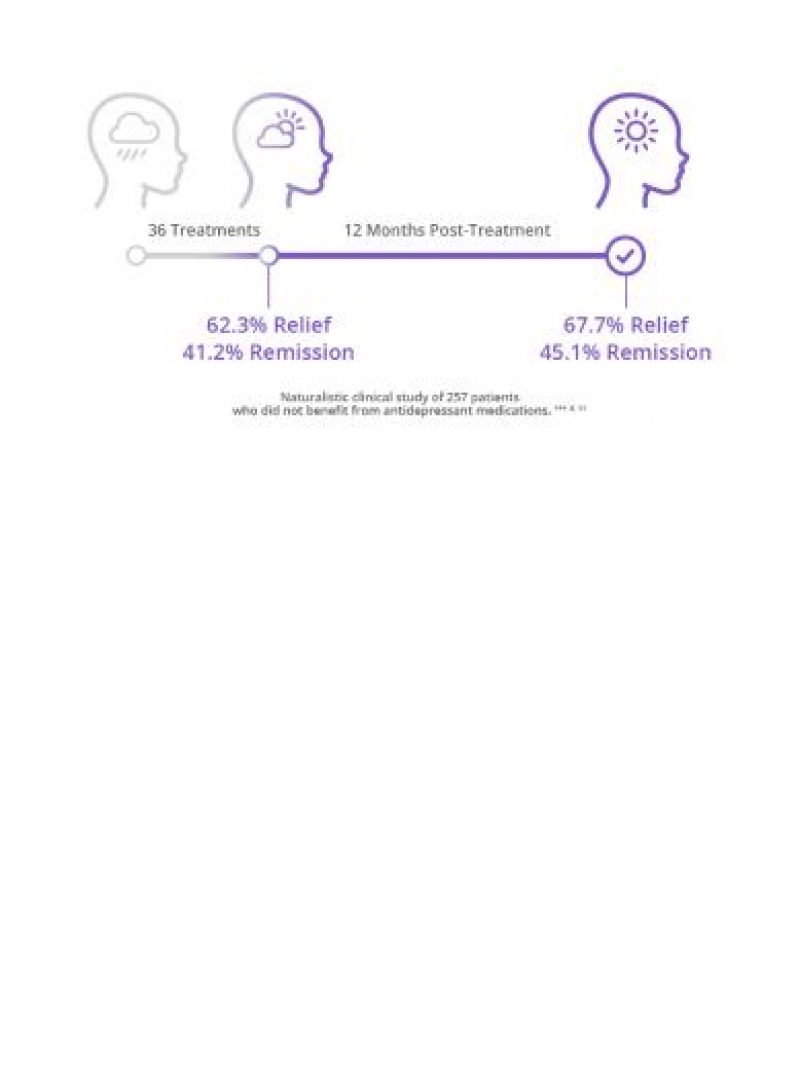
Proven Depression Relief That Lasts
Studies show that with each unsuccessful medication attempt, chances of abandoning treatment increase. However NeuroStar TMS has response and remission rates that cannot be denied, including durability for up to 12 months.8,11
See Our Clinical Data
Revolutionize Your Practice
With industry-leading innovations like TrakStar, D-Tect, FastMT and more, NeuroStar is constantly pushing the envelope for better patient outcomes, more efficient processes, and a more seamless treatment experience for both patients and providers.
Explore Our Technology
Learn How TMS Works for Mental Health

Unlock Your Practice’s Potential With TrakStar
Begin Your Journey with NeuroStar Today


