Results
Changing Lives, With the Evidence to Prove It For patients with drug-resistant major depression, the life-changing power of NeuroStar® TMS is clear. It’s backed by substantial data, including the world’s largest depression Outcomes Registry**.
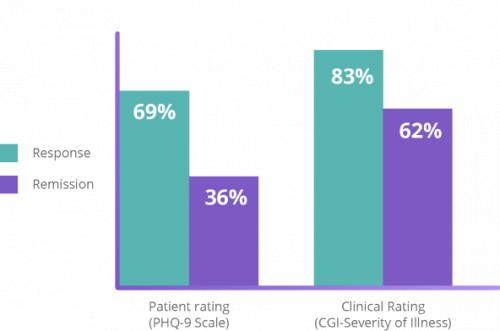
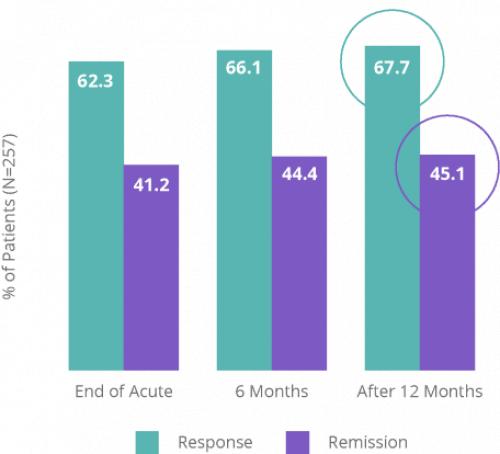
Proven Depression Relief That Lasts
More Clinical Data than Any Other TMS Provider

Real-World Trials
NeuroStar’s safety and efficacy is backed by the largest clinical data set of any TMS system for depression.2 The clinically significant antidepressant effect of NeuroStar Advanced Therapy was demonstrated in two large, multisite, randomized, controlled trials.11,19 NeuroStar’s efficacy was also demonstrated in a multisite, real-world, open-label clinical trial in which patients received an acute NeuroStar treatment course.7,11,12,15,17,19

4x Remission Rate
Clinical results demonstrated an improvement in depression symptoms. In an NIMH-funded, independent, randomized, controlled trial, patients treated with NeuroStar Advanced Therapy were four times more likely to achieve remission compared to patients receiving sham treatment.11 Significant improvement from baseline with NeuroStar Advanced Therapy was observed as early as 2 weeks after treatment and at 4 and 6 weeks (P=0.0006).4
65 Clinical Studies
NeuroStar’s commitment to advancing the science of TMS and neurohealth has been demonstrated in over 65 investigator-initiated clinical studies with more than 1,900 patients.
If you’re interested in conducting an Investigator Initiated Trial, please contact Neuronetics Customer Service at 877-600-7555.

Numerous Publications
NeuroStar is proven in scientific publicati ons and presentations. Click here for links to publications related to the safety and efficacy of NeuroStar Advanced Therapy.

Numerous Publications
NeuroStar is proven in scientific publications and presentations. Click here for links to publications related to the safety and efficacy of NeuroStar Advanced Therapy.
The Best Evidence Is a Changed Life
MDD patients often go through years of trial and error with different medications. For those people, the life-changing power of NeuroStar can best be grasped through their own words.

MLB’s Drew Robinson — a TMS Journey
Drew Robinson had reached the peak, playing baseball at the highest level, but inside Drew was suffering. He thought he was out of options, and tried to take his own life.
Drew's NeuroStar JourneyJoin the NeuroStar Community