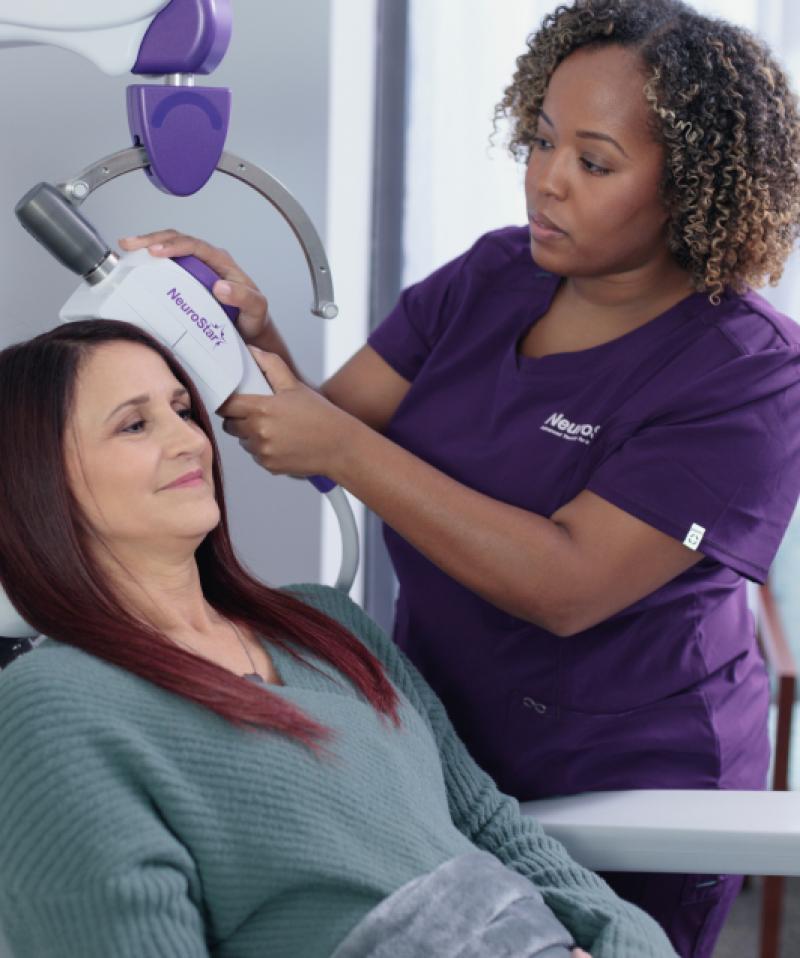
Technology
NeuroStar® Is the TMS Innovation Leader NeuroStar is continually innovating for the sake of both your patients and practice, reducing the time it takes to get patients into and through treatment and getting more patients the help they need.
One Coil, Multiple Treatments — the Most Versatile Coil in TMS
Treat multiple diagnoses with no additional upgrades.
In addition to MDD, NeuroStar TMS is now FDA-cleared for the treatment of both obsessive-compulsive disorder (OCD) and anxious depression (major depression disorder comorbid with anxiety). Thanks to our industry-leading platform, existing NeuroStar providers can use the same system for all approved treatments with no additional upgrades or purchases.
Right Dose, Right Place, Every Time
NeuroStar’s patented Contact Sensing system provides real-time feedback in three dimensions and delivers an alert if contact with the coil is compromised, ensuring your patients are receiving an accurate dosage at the right location, every time.2
Reduce MT Time by up to 40%2
NeuroStar helps you get more patients into and through treatment faster, with innovations that optimize the process for Motor Threshold (MT) determination like:
- MT Cap, Fast MT™, and D-Tect – Allowing one treater to identify Motor Threshold in a fraction of the time of other systems, decreasing the time your patients spend in the chair, and increasing the number of patients you can treat.
- 3-D Laser-Guided Six Point Coordinate System – Ensuring returning patients get treated quickly and accurately.

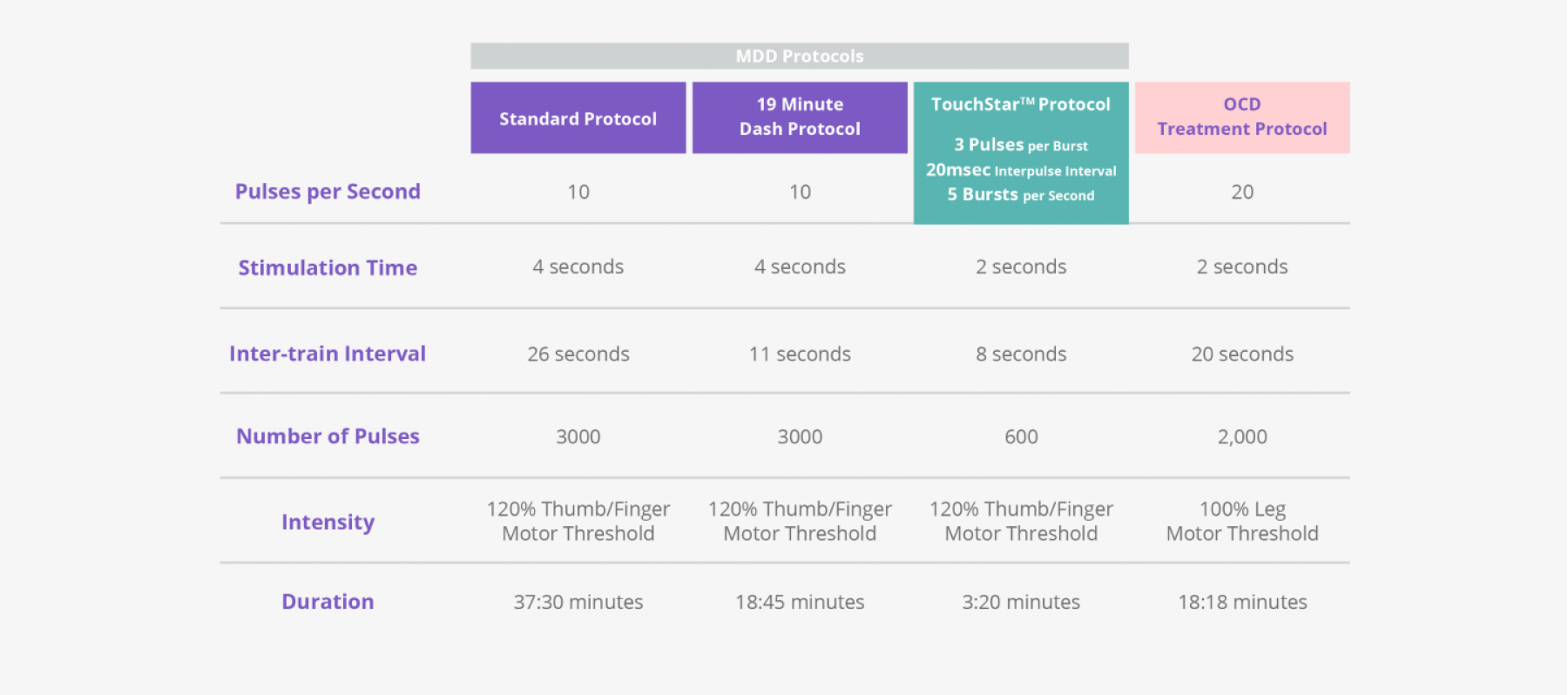
TouchStar™ Theta Burst
A new way to dramatically reduce your patients’ treatment session times, TouchStar™ Theta Burst with Contact Sensing is a NeuroStar treatment protocol that involves higher-frequency pulses than our standard protocols. This results in treatment sessions being reduced to around 3 minutes.
NeuroStar TMS is now FDA-cleared for OCD and Anxious Depression
Thanks to our industry-leading platform, adding new treatment protocols is as simple as a software update, putting new treatment possibilities right at your fingertips.
It’s just one more way NeuroStar is constantly innovating for the benefit of your patients and your practice.
Frequently Asked Questions About NeuroStar TMS
Is NeuroStar safe and does it have any side effects?
Do NeuroStar treatments hurt?
Can NeuroStar cause seizures?
Patients should be carefully monitored for worsening symptoms and/or signs or symptoms of suicidal behavior and/or unusual behavior. Families and caregivers should also be aware of the need to observe patients and notify their treatment provider if symptoms worsen.2
NeuroStar Advanced Therapy should not be used with patients who have non-removable conductive metal in or near the head.
Reach out Today and Accelerate Your Practice
Contact a representative today to see how NeuroStar can take your practice to a new level.