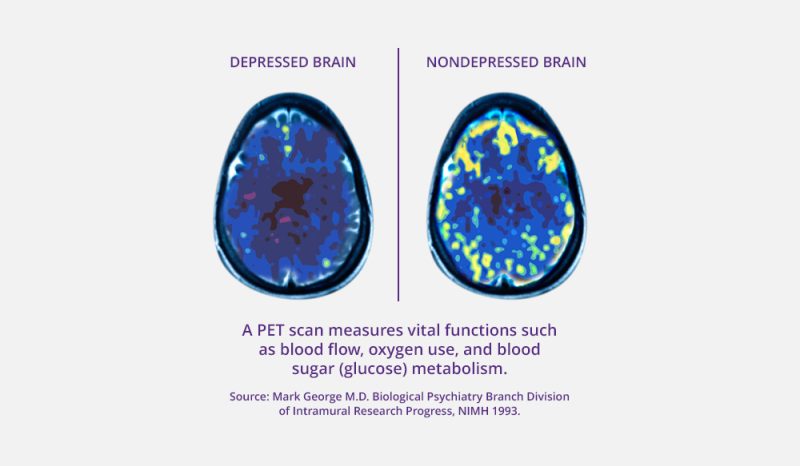
Indication:
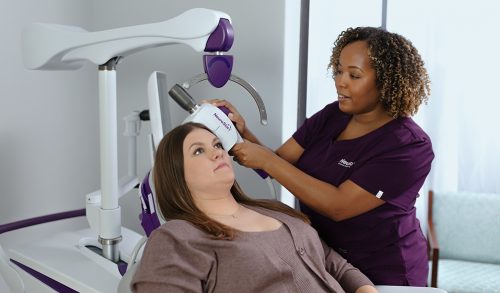
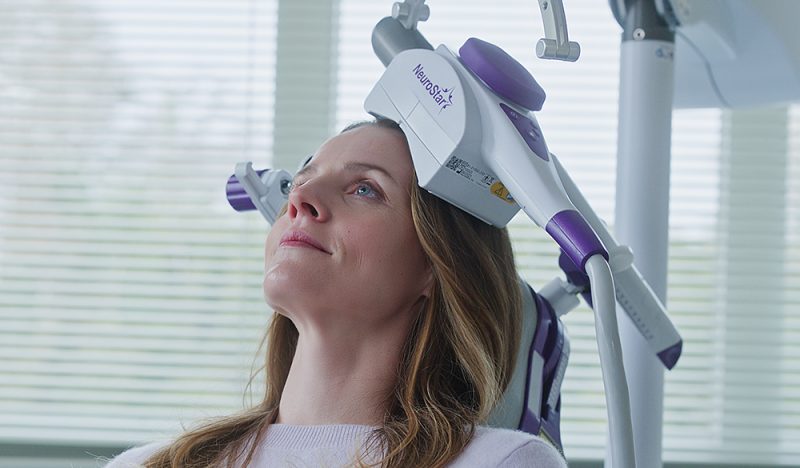
NeuroStar TMS Therapy is indicated for the treatment of Major Depressive Disorder in adult patients who have failed to receive satisfactory improvement from prior antidepressant medication in the current episode.
NeuroStar TMS Therapy is only available by prescription. A doctor can help decide if NeuroStar TMS Therapy is right for you.
Contraindications:
The NeuroStar TMS Therapy System is contraindicated for use in some situations. All patients must be screened for the following contraindications:
Metallic Objects in or near the Head:
The NeuroStar TMS Therapy System is contraindicated for use in patients who have conductive, ferromagnetic, or other magnetic-sensitive metals implanted in their head within 30 cm of the treatment coil. Examples include cochlear implants, implanted electrodes/stimulators, aneurysm clips or coils, stents, bullet fragments, jewelry and hair barrettes. Failure to follow this restriction could result in serious injury or death.
NOTE Removable objects that may be affected by the magnetic field should be removed from the patient before treatment to prevent possible injury. (Examples include jewelry and hair barrettes). Once these objects are removed, NeuroStar TMS Therapy is not contraindicated for these patients.
NOTE Examples of metallic objects in or near the head that are acceptable under certain conditions include standard amalgam dental fillings, single post dental implants, and dental bridge work.
Implanted Stimulator Devices in or near the Head:
The NeuroStar TMS Therapy System is contraindicated for use in patients who have active or inactive implants (including device leads), including deep brain stimulators, cochlear implants, and vagus nerve stimulators. Contraindicated use could result in serious injury or death.
Warnings:
NeuroStar TMS Therapy has not been evaluated in patients with psychoses or with psychiatric emergencies where a rapid clinical response is needed, such as marked physical deterioration, catatonia, or immediate suicide risk. Use of NeuroStar TMS Therapy in the treatment of these patients is not recommended since rapid onset of effect in these high-risk populations has not been established.
Following acute treatment with NeuroStar TMS Therapy, patients will need to be monitored and may need to resume antidepressant medications. This device has not been evaluated for durability of antidepressant effect in controlled clinical trials.
Worsening Depression or Suicidality
Patients who have Major Depressive Disorder may experience worsening of their depression and/or the emergence of suicidal ideation and behavior (suicidality) or unusual changes in behavior, whether or not they are being treated with an antidepressant, and this risk may persist until significant remission of symptoms occurs.
Observe patients undergoing treatment for Major Depressive Disorder closely for worsening symptoms and signs of suicidal behavior and/or unusual behavior. If worsening of symptoms continues, consideration should be given to changing the therapeutic regimen, including discontinuation of treatment with the NeuroStar TMS Therapy System. Families and caregivers should also observe patients and notify the treatment provider if symptoms worsen.
Effects on Medical Devices Containing Electronics or Ferromagnetic Material
- Implants Controlled by Physiologic Signals
The NeuroStar TMS Therapy System should be used with caution in patients who have an implanted device that is activated or controlled in any way by physiologic signals, even if the device is located outside the 30 cm distance. This includes patients with pacemakers and implantable cardioverter defibrillators (ICDs) or patients using wearable cardioverter defibrillators (WCDs) even if the device is removed due to the potentially unstable cardiac condition of such patients. Failure to follow this restriction could result in serious injury or death.
- Implants Not Controlled by Physiologic Signals
Patients who have implanted devices or metallic objects located in areas outside the 30 cm distance from the treatment coil may receive NeuroStar TMS Therapy. However, care must be taken by the NeuroStar TMS Therapy System operator to ensure that the treatment coil is never placed within 30 cm of these implants. Otherwise, serious injury could result. (Examples of these devices include staples and implanted insulin pumps.)
- Wearable or Removable Devices or Objects
If patients have removable devices or objects that may be affected by the magnetic field, the device(s) should be removed from the patient area before treatment to prevent possible injury to the wearer or damage to the device. (Examples include wearable monitors, bone growth stimulators, earrings, hearing aids, eyeglasses, jewelry, hair barrettes, cell phones, MP3 players, etc.)
Risk of Seizure
Generalized seizures have been reported with the use of TMS in the clinical trial literature. No seizures were reported with use of the NeuroStar TMS Therapy System in over 10,000 treatment sessions in trials conducted prior to FDA clearance of the NeuroStar TMS Therapy System. Since the introduction of the NeuroStar TMS Therapy System into clinical practice, seizures have been rarely reported. The estimated risk of seizure under ordinary clinical use is approximately 1 in 30,000 treatments (0.003% of treatments) or 1 in 1000 patients (0.1% of patients). Nevertheless, the NeuroStar TMS Therapy System should be used with caution in patients who have a history of seizures, or a potential for alteration in seizure threshold, as stated below.
Patients at potential increased risk of seizure include those who have:
- History (or family history) of seizure or epilepsy
- History of stroke, head injury, severe headaches, or unexplained seizures
- Presence of other neurological disease that may be associated with an altered seizure threshold (such as CVA, cerebral aneurysm, dementia, increased intracranial pressure, head trauma, or movement disorder)
- Concurrent medication use such as tricyclic antidepressants, neuroleptic medications, or other drugs that are known to lower the seizure threshold
- Secondary conditions that may significantly alter electrolyte balance or lower seizure threshold
- No quantifiable motor threshold such that TMS dosage cannot be accurately determined
Cautions:
- The acute effectiveness of NeuroStar TMS Therapy has not been established beyond a six-week treatment course for MDD.
- NeuroStar TMS Therapy has not been studied as an adjunct to antidepressant treatment in controlled trials; it has been administered safely in the presence of antidepressant medication.
The patient and the operator of the NeuroStar TMS Therapy System must always wear earplugs or similar hearing protection devices with a rating of 30 dB of noise reduction during treatment. When used with appropriate hearing protection, NeuroStar TMS Therapy did not have an effect on auditory threshold.
Longer-term effects of exposure to the NeuroStar TMS Therapy System magnetic field are not known. Experimental and observational evidence indicates that exposure to the type of magnetic fields produced by the NeuroStar TMS Therapy System coil does not present any significant risk of acute or long-term adverse effects.
Adverse Events:
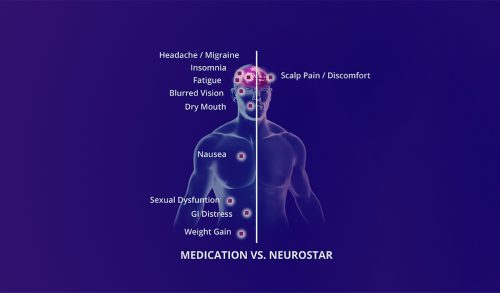
The most common adverse events reported in controlled clinical trials of the NeuroStar TMS Therapy System were application site pain and headache. Application site pain was the most frequently reported device-related adverse event with greater frequency in the active TMS treatment group as compared to sham TMS. Headache was reported by about half of patients and nearly equally in both active TMS and sham TMS treatment groups. In general, application site pain and headache were transient and dissipated rapidly with time. These adverse events were graded as mild to moderate in severity for the majority of patients.